Research & Development
A Long-Term Commitment to Health Innovation
At Hovid, we understand that the battle against diseases is an ongoing challenge. We believe that passion drives every breakthrough, empowering us to push the boundaries of healthcare services. Innovation is at the heart of our mission to develop new, patented formulations that contribute to the prevention and treatment of diseases.
Fighting to Improve People’s Health
Professional Scientists & Laboratory Executives
Bioequivalence (BE) Studies completed
Products in the R&D Pipeline
Clinical Trials
Our Qualified Facilities
Tailored to Meet Customer Needs
Hovid’s R&D division, known as Attest Research Sdn Bhd, focuses on stability and bioequivalence (BE) studies—critical for ensuring the quality and consistency of our products. Recognized by the Ministry of Health Malaysia as one of the few centers authorized to conduct BE studies, we ensure that all Hovid products meet the highest levels of quality, efficacy, and safety.
To meet the increasing demand for BE studies, we have established a large, advanced facility in Bayan Lepas, Penang, equipped with cutting-edge clinical technologies. This facility also provides a comfortable environment for volunteers participating in our studies. Additionally, another dedicated R&D team operates from our manufacturing plant in Chemor, Perak, further supporting and driving these essential studies.

What we do

Product Development
Product Development
- Solid, semi-solid and liquid oral dosage forms
- Topical preparations, including emulsions, suspensions, creams and ointments
- Soft gelatin preparations

Drug Delivery System Innovation
Drug Delivery System Innovation
- Controlled-release formulations
- Enhanced oral bioavailability formulations
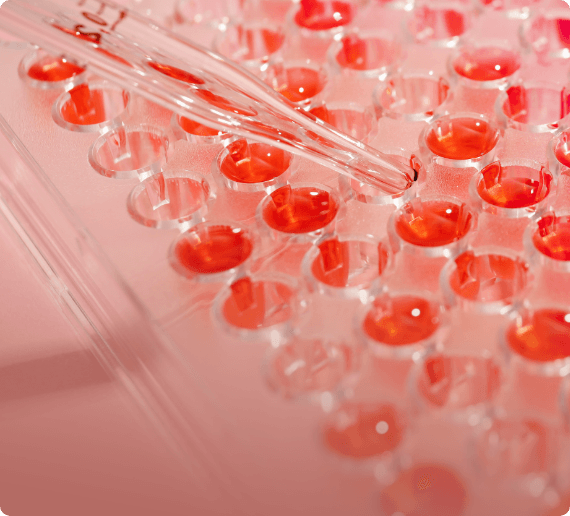
Stability Studies & Testing
Stability Studies & Testing
- 25°C ± 2°C / 60% ± 5% RH
- 30°C ± 2°C / 75% ± 5% RH
- 40°C ± 2°C / 75% ± 5% RH

Clinical Services
Clinical Services
- Bioequivalance (BE) studies
- Bioavailability (BA) studies
- Efficacy studies