Manufacturing
At Hovid, we are committed to delivering high-quality pharmaceutical products that meet the evolving needs of consumers worldwide. Our manufacturing facilities adhere to global standards, ensuring both product safety and efficiency in every step of production.
20-Acre Chemor Plant
The Core of Our Manufacturing Operations

Hovid’s 20-acre manufacturing plant in Chemor, Perak, serves as a cornerstone of our pharmaceutical production capabilities. Equipped with advanced technology and operating under globally recognized standards, this facility ensures consistent delivery of high-quality pharmaceutical products to meet the demands of healthcare providers and patients worldwide.
With an emphasis on sustainability and innovation, the Chemor facility is designed to support Hovid’s long-term growth, enabling the development of groundbreaking pharmaceutical solutions while maintaining environmental responsibility.
List of manufacturing activities

Oral solid dosage forms
i.e. tablets & hard gelatin capsules

R&D headquarters

Walk-in stability study rooms

Microlab

Softgel packing

Supporting Excellence
Ipoh Headquarters
In addition to the Chemor plant, our 3-acre headquarters in Ipoh plays a vital role in overseeing production, corporate operations, and administrative functions. Together, these facilities enable us to deliver innovative healthcare solutions with a commitment to excellence.
List of manufacturing activities

Soft gel encapsulation

Oral liquid dosage form

Herbal tea plant

Topical preparation

Penicillin production
Global Certifications and Standards
Our manufacturing processes comply with regulatory best practices and the highest international standards.

Pharmaceutical Inspection Co-operation Scheme (PIC/S)

cGMP (current Good Manufacturing Practices)

GLP (Good Laboratory Practices)

TGA (Therapeutic Goods Administration)

EU GMP certification (Estimated 2025)

ISO/IEC 17025:2005 Certification
Innovative & Diversified Portfolio
With a portfolio of over 400 pharmaceutical products, Hovid provides high-quality solutions in a wide range of categories, including Generic Pharmaceuticals, OTC Supplements, Disinfectant Solutions and Medical devices.
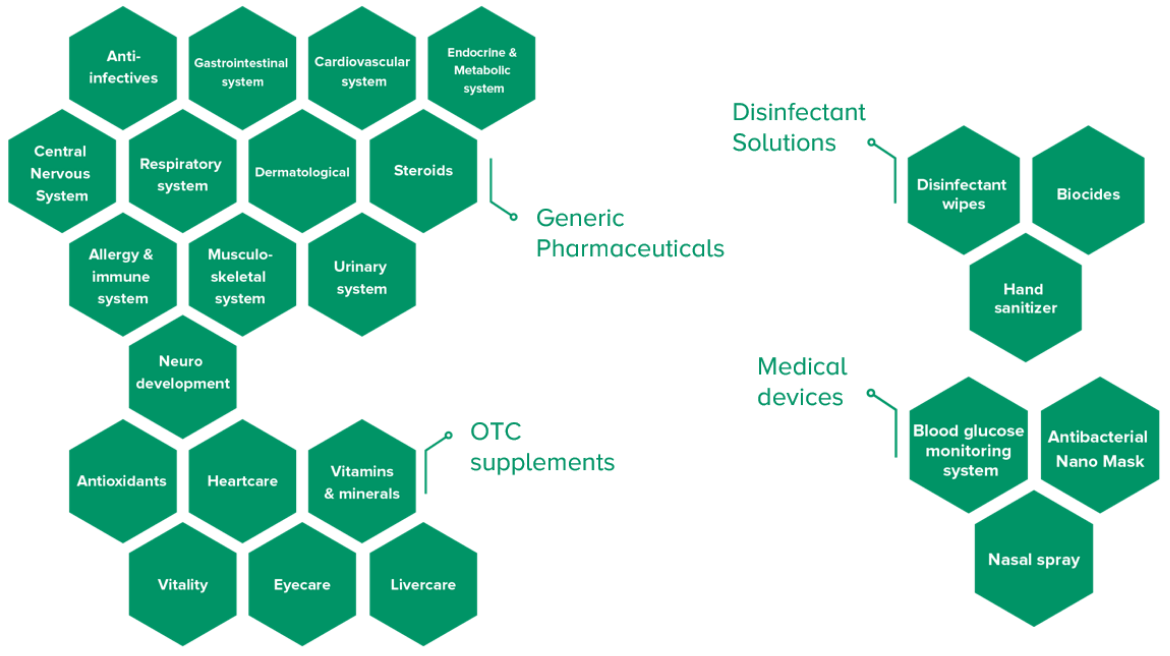
Our products are developed and manufactured with a focus on stability, quality, and affordability, ensuring they meet the needs of patients at competitive prices.
Contract Manufacturing Services
We offer contract manufacturing services for various pharmaceutical products, ensuring efficiency and quality at every stage. Our laboratory and Bioequivalence (BE) studies provide the expertise needed for optimal product development, stability, and market readiness.
Continuous Development to Meet Consumer Needs
Hovid continues to evolve its portfolio by developing new products that address the changing needs of consumers. We focus on innovative research and development to deliver effective, safe, and affordable pharmaceutical solutions that improve lives.

